
PEM electrolyzer cell is an advanced patented product, which is Low voltage and higher efficiency, energy-saving and of environmental protection , producing hydrogen and oxygen through the electrolysis of pure water (without adding alkali).
PEM electrolyzer cell is an advanced patented product, which is Low voltage and higher efficiency, energy-saving and of environmental protection , producing hydrogen and oxygen through the electrolysis of pure water (without adding alkali).
That’s PEM technology. The SPE electrodes, as the core of cell , are highly active catalytic electrode with nearly zero distance between the electrodes , which is formed by integrating composite catalyst with and ion membrane with high electrolytic efficiency.
The performance and life of PEM electrolyzer are closely related to product design, assembly process, usage conditions, etc.




| Capacity | 3000ml/min |
| Voltage | 34V |
| Current | 52A |
| Number of plates | 10pcs |
| Water quality requirements | deionized water or distilled water |
| Core Components | Dupont N117, Titanium mesh |
| Technology | PEM SPE Technology |
| Dimension | 118*128*141mm |




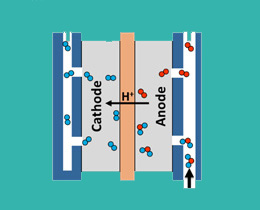
Working Principle of electrolyzer cell
The proton exchange membrane (PEM), which only allows water and positive ions to cross between compartments. The membrane also serves as the electrolyte in the cell, eliminating the need for hazardous liquid electrolytes such as concentrated potassium hydroxide. PEM water electrolysis simply splits pure deionized water (H2O) into its constituent parts, hydrogen (H2) and oxygen (O2), on either side of this membrane. When a DC voltage is applied to the electrolyzer, water fed to the anode, or oxygen electrode, are oxidized to oxygen and protons, while electrons are released. The protons (H+ ions) pass through the PEM to the cathode, or hydrogen electrode, where they meet electrons from the other side of the circuit, and are reduced to hydrogen gas. The two reactions that occur in the cell are as
follows:
2H2O -> 4H+ + 4e- +O2
4H+ + 4e- -> 2H2
Thus, the only possible components of the streams are hydrogen, oxygen and water moisture, as shown in the following picture:
Using Tips
1.Use pure water,deionized water or distilled water for the machine.
2.The customer should be pay attention to the water level.It is forbidden to operate it for the shortage of water to prevent the electrolytic cell from damage.